Polypropylene is one of the most widely used plastics in the world, known for its versatility, lightweight nature, and durability. It can be found in a variety of products, from everyday household items like food containers and packaging materials to industrial applications such as automotive parts and textiles. But how exactly is polypropylene made? The answer lies in a fascinating and complex process that starts with crude oil. Crude oil, a natural fossil fuel, serves as the primary raw material for the production of many types of plastics, including polypropylene. This transformation from a thick, dark liquid to a clear, durable plastic involves several stages of chemical processing and refining. In this article, we will take a detailed look at how polypropylene is made from crude oil, starting from its extraction, refining, chemical reactions, and final polymerization.
1. Understanding Crude Oil and Its Components
Crude oil is a naturally occurring, unrefined petroleum product composed of hydrocarbon deposits and other organic materials. It is found deep underground, extracted using various drilling methods, and serves as the raw material for numerous products, including fuels, lubricants, and plastics.
What Are Hydrocarbons
Hydrocarbons are the building blocks of crude oil and consist of hydrogen and carbon atoms. They come in various forms, including alkanes, alkenes, and aromatic hydrocarbons. These molecules are essential for producing synthetic materials like polypropylene. The process of converting crude oil into plastics involves breaking down these hydrocarbons and rearranging them into new chemical structures.
2. Refining Crude Oil: The First Step
Before crude oil can be used to produce polypropylene, it must first undergo refining. The refining process separates the complex mixture of hydrocarbons found in crude oil into simpler fractions based on their boiling points.
Fractional Distillation
The primary method used for refining crude oil is fractional distillation. In this process, the crude oil is heated in a large distillation column, causing it to vaporize. As the vapor rises through the column, different hydrocarbon fractions condense at different levels based on their boiling points.
The main fractions obtained from fractional distillation include:
- Gases (e.g., methane, ethane)
- Naphtha
- Kerosene
- Diesel
- Heavy fuel oil
- Residuum
Among these fractions, naphtha plays a critical role in the production of polypropylene, as it contains the alkanes and alkenes needed for further chemical processes.
See also: What Does It Mean to Refine Crude Oil?
3. Cracking: Breaking Down Hydrocarbons
After the fractional distillation of crude oil, the naphtha fraction undergoes a process called cracking to break down the larger hydrocarbon molecules into smaller, more useful ones. This step is essential for producing the basic building blocks of polypropylene.
Types of Cracking
There are two main types of cracking:
Thermal Cracking: Uses high temperatures and pressures to break down large hydrocarbons into smaller ones.
Catalytic Cracking: Involves the use of catalysts (substances that speed up chemical reactions) to lower the required temperature and pressure, making the process more efficient.
For polypropylene production, a specific type of catalytic cracking known as “steam cracking” is often used. During steam cracking, naphtha is heated in the presence of steam, resulting in the formation of lighter hydrocarbons such as ethylene and propylene, which are gases at room temperature.
4. Producing Propylene: The Key Ingredient
Propylene is the primary monomer (a molecule that can chemically bond to other molecules) needed to create polypropylene. It is derived from the cracking process, where hydrocarbons from the naphtha fraction are broken down into smaller molecules.
The Role of Steam Cracking
During steam cracking, the naphtha is exposed to very high temperatures (around 800-900°C or 1,470-1,650°F) in a cracking furnace. This process breaks down the large hydrocarbons into smaller alkenes such as ethylene and propylene. The propylene is then separated from the other by-products using a series of distillation steps to purify it for the next stage.
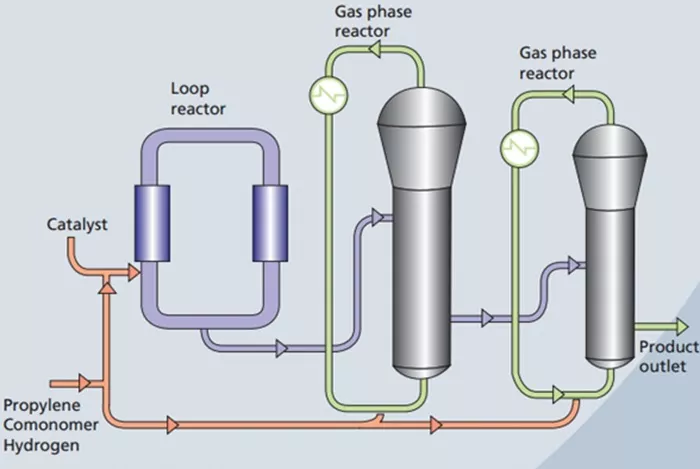
5. Polymerization: Turning Propylene into Polypropylene
The purified propylene monomers are now ready for polymerization, a chemical reaction that combines these small molecules into long chains to form a polymer. The resulting polymer is polypropylene.
Types of Polymerization
There are different methods of polymerization, but for polypropylene production, the most common is Ziegler-Natta polymerization or metallocene catalysis. Both methods use catalysts to control the reaction and produce a polymer with specific properties.
Ziegler-Natta Polymerization: Uses a special catalyst developed by chemists Karl Ziegler and Giulio Natta, which enables the polymerization of propylene under relatively mild conditions. This method produces high-quality polypropylene with a consistent structure.
Metallocene Catalysis: Employs metallocene catalysts, which provide better control over the polymer’s structure, allowing for the production of polypropylene with enhanced properties such as improved strength and flexibility.
6. Modifying and Processing Polypropylene
Once the polymerization is complete, the polypropylene undergoes various modifications to improve its properties or meet specific requirements for different applications.
Adding Stabilizers and Additives
To enhance polypropylene’s durability, stabilizers, antioxidants, and other additives may be mixed in. These substances protect the plastic from degradation caused by UV radiation, heat, and chemical exposure.
Pelletizing
The modified polypropylene is then extruded into long strands, which are cooled and chopped into small pellets. These pellets are the final form of polypropylene that manufacturers buy and use to produce different products.
See also: How Long Does It Take to Process Crude Oil into Gasoline?
7. Applications of Polypropylene
Polypropylene is a versatile plastic that finds applications in numerous industries. Some of the common uses include:
Packaging: Used in food containers, bottle caps, and plastic wraps due to its resistance to moisture and chemicals.
Automotive Parts: Polypropylene is lightweight and durable, making it ideal for car bumpers, interior panels, and battery cases.
Textiles: Woven into fibers for rugs, carpets, and upholstery due to its strength and stain resistance.
Medical Devices: Used in disposable syringes, pill bottles, and surgical instruments due to its non-reactivity and ease of sterilization.
Household Items: Commonly found in products such as storage bins, toys, and furniture components.
8. Environmental Considerations
While polypropylene is a valuable material, it is essential to consider the environmental impact of its production and disposal.
Recycling Challenges
Polypropylene is recyclable, but the recycling rate remains relatively low compared to other plastics. This is due to difficulties in sorting and the lower value of recycled polypropylene.
Efforts Towards Sustainability
To address environmental concerns, research is being conducted to develop bio-based polypropylene made from renewable resources, as well as chemical recycling techniques that break down polypropylene back into its original monomers for reuse.
Conclusion
Polypropylene production is a complex process that begins with crude oil extraction and involves several stages, including refining, cracking, polymerization, and modification. Starting from crude oil, a mixture of hydrocarbons, the journey to create polypropylene involves breaking down the oil into simpler components and then reassembling those components into a versatile and widely used plastic. Understanding this process gives insight into how a raw natural resource can be transformed into a synthetic material with countless applications. Although polypropylene offers many benefits, the environmental implications of its production and disposal cannot be overlooked. Sustainable practices and advancements in recycling technology are essential to minimize the impact of polypropylene on the planet.
Related Topics: