Crude oil is a complex mixture of various hydrocarbons, which are molecules composed of hydrogen and carbon. These hydrocarbons exist in different forms and sizes, ranging from light gases to heavy, thick oils. One of the most important processes in the oil industry is separating these hydrocarbons to create useful products, such as gasoline, diesel, jet fuel, and petrochemicals. This process is vital for the production of energy and the manufacturing of numerous products used in everyday life.
The name of the process used to separate hydrocarbons from crude oil is called distillation. Specifically, it is referred to as fractional distillation. This technique takes advantage of the different boiling points of the various components in crude oil. In this essay, we will explore what fractional distillation is, how it works, and why it is essential in the oil refining industry. We will also discuss the different fractions produced through this process and how they are used in various industries.
Introduction to Crude Oil and Hydrocarbons
Crude oil, also known as petroleum, is a naturally occurring liquid found beneath the Earth’s surface. It is primarily composed of hydrocarbons, which are organic compounds made up of hydrogen and carbon atoms. These hydrocarbons vary greatly in size and complexity, ranging from small molecules like methane (CH4) to much larger, more complex molecules like asphaltenes, which are solid at room temperature.
Crude oil also contains other elements such as sulfur, nitrogen, oxygen, and trace metals, but hydrocarbons make up the majority of its composition. The challenge in refining crude oil is to separate these hydrocarbons into different categories or “fractions” that can be used for different purposes.
Crude oil does not consist of just one type of hydrocarbon but a wide range of compounds. These compounds are classified based on their molecular size and boiling points. Some hydrocarbons are gases at room temperature, while others are liquid or solid. To make these hydrocarbons useful for various applications, they need to be separated through a refining process.
The Process of Fractional Distillation
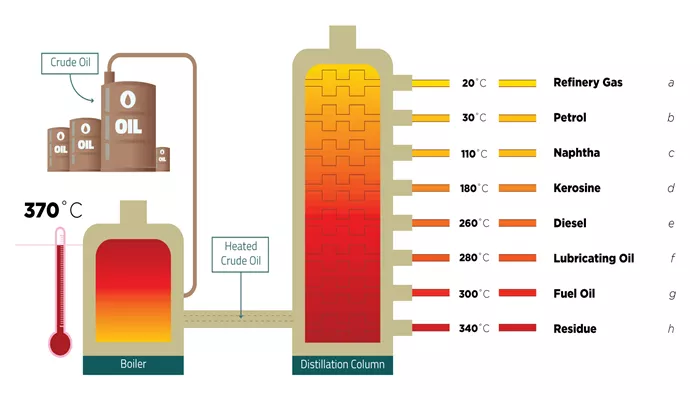
Fractional distillation is the method used to separate the various components of crude oil. It is a physical process that relies on the principle that different substances have different boiling points. When crude oil is heated, it begins to vaporize. The vapor is then cooled and condensed into liquid fractions based on their boiling points.
The crude oil is first heated in a furnace to temperatures of around 350°C (662°F) or higher. At this temperature, the different hydrocarbons in the crude oil start to evaporate, with lighter hydrocarbons vaporizing at lower temperatures and heavier ones remaining as liquids. The vaporized hydrocarbons are then passed into a distillation column, also known as a fractionating column.
The distillation column is a tall, vertical structure filled with trays or packing material that provides surface area for condensation. As the vapor rises through the column, it cools and condenses at different levels based on the boiling point of the hydrocarbons. The heavier, high-boiling hydrocarbons condense at the bottom of the column, while lighter hydrocarbons condense higher up.
Each tray in the column collects a different fraction, which can be further processed into useful products. The process allows for the separation of crude oil into its various components, each of which has its own unique properties and uses.
The Different Fractions Produced by Fractional Distillation
Fractional distillation results in the separation of crude oil into several distinct fractions, each with different boiling ranges and uses. These fractions are classified based on their molecular weight, size, and boiling points. The main fractions obtained from crude oil include:
1. Gases (Light Hydrocarbons)
At the top of the distillation column, the lightest hydrocarbons are collected. These include gases like methane, ethane, propane, and butane. These gases are used as fuel for heating, cooking, and in the production of chemicals. Propane and butane, for example, are often used as liquefied petroleum gas (LPG) for household and industrial purposes.
2. Gasoline (Petrol)
Gasoline is one of the most valuable products derived from crude oil. It is collected just below the gas fraction and is used as fuel for vehicles. Gasoline typically consists of hydrocarbons with between 5 and 12 carbon atoms. The exact composition of gasoline can vary, but it is primarily made up of alkanes, cycloalkanes, and aromatic hydrocarbons.
3. Kerosene (Jet Fuel)
Kerosene is collected just below the gasoline fraction. It is a type of jet fuel used in aviation. Kerosene has a higher boiling point than gasoline, and its hydrocarbons typically contain between 12 and 16 carbon atoms. Kerosene is also used in heating and lighting applications in some parts of the world.
4. Diesel Fuel
Diesel fuel is heavier than kerosene and is collected lower down in the distillation column. It is used as fuel in diesel engines, which are commonly found in trucks, buses, and other heavy-duty vehicles. Diesel fuel contains hydrocarbons with between 16 and 20 carbon atoms and has a higher energy density than gasoline, making it more efficient for larger vehicles.
5. Lubricating Oils
Lubricating oils, such as motor oil, are heavier fractions that are collected further down the column. These oils are used to lubricate engine parts and machinery, reducing friction and wear. The hydrocarbons in lubricating oils typically contain between 20 and 30 carbon atoms. These oils are often refined and processed to meet specific viscosity and performance requirements.
6. Fuel Oil
Fuel oil, which is used for heating and power generation, is collected near the bottom of the distillation column. This fraction is much heavier and thicker than the lighter oils, and it contains hydrocarbons with more than 30 carbon atoms. Fuel oil is used in industrial boilers, ships, and large power plants.
7. Bitumen (Asphalt)
At the very bottom of the distillation column, the heaviest and thickest fraction is collected, which is known as bitumen or asphalt. Bitumen is a viscous, tar-like substance used primarily in road construction and paving. It is also used in the production of roofing materials and waterproofing products.
The Role of Catalytic Cracking and Other Refining Processes
While fractional distillation is the primary method of separating hydrocarbons from crude oil, it is not the only process involved in refining crude oil. Many of the heavier fractions, such as fuel oil and bitumen, are not ideal for many industrial applications. To make these fractions more useful, additional refining processes, such as catalytic cracking, hydrocracking, and alkylation, are used.
Catalytic cracking is a process in which heavy hydrocarbons are broken down into lighter, more useful products like gasoline and diesel. This is achieved by using a catalyst and high heat to break the chemical bonds of large hydrocarbon molecules. The resulting lighter hydrocarbons can then be blended into gasoline or diesel fuel, depending on their properties.
Hydrocracking is similar to catalytic cracking but involves the use of hydrogen gas under high pressure. This process helps improve the quality of the products by removing impurities such as sulfur and nitrogen.
Alkylation is another process used to produce high-octane gasoline by combining smaller molecules, such as isobutane, into larger molecules. This process helps improve the performance of gasoline, making it more efficient and less prone to engine knocking.
The Importance of Distillation in the Oil Industry
Fractional distillation is a crucial step in the oil refining process because it separates the complex mixture of hydrocarbons into individual components that can be used for different applications. Without distillation, the various valuable products derived from crude oil would not be able to be isolated and refined.
In addition to producing fuels like gasoline, diesel, and jet fuel, fractional distillation also allows for the production of important by-products like petrochemicals, which are used in the manufacturing of plastics, fertilizers, and other chemicals. The ability to separate and refine crude oil into these products is essential for powering the global economy and supporting a wide range of industries.
Conclusion
Fractional distillation is the process used to separate hydrocarbons from crude oil. By heating crude oil and allowing the various components to condense at different levels in a distillation column, this process enables the oil industry to produce a wide range of valuable products, including gasoline, diesel, jet fuel, and petrochemicals. Although fractional distillation is the primary method of separating crude oil, additional processes like catalytic cracking and hydrocracking are often used to improve the quality and yield of these products. The importance of distillation in the oil industry cannot be overstated, as it enables the extraction of essential fuels and chemicals that power the world’s economy.
Related Topics:
Oil Prices Climb On Positive Chinese Factory Data And Middle East Tensions