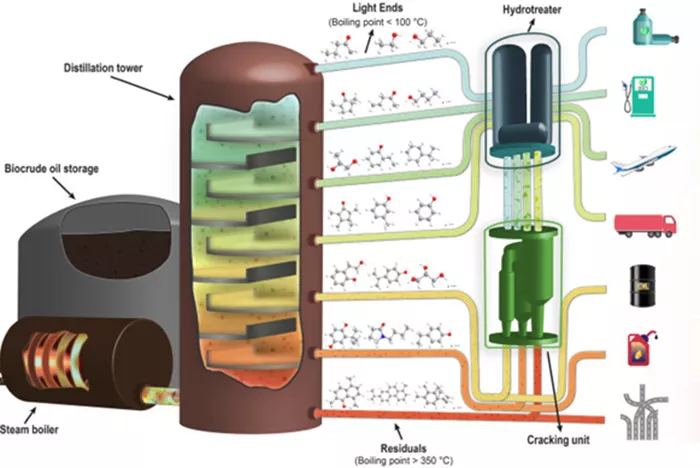
Crude oil is one of the most important natural resources in the world, as it serves as the foundation for producing numerous fuels and chemicals. However, crude oil in its raw form is a complex mixture of hydrocarbons, and its various components must be separated before they can be used. One of the most valuable products extracted from crude oil is gasoline, which powers the majority of vehicles worldwide. The process of separating gasoline from crude oil is a sophisticated and vital step in petroleum refining. Fractional distillation is the most viable and widely used method for achieving this separation. It leverages the differences in boiling points of hydrocarbons, allowing gasoline to be effectively isolated from other components in crude oil. In this article, we will explore the fractional distillation process in detail, along with additional post-distillation steps that further refine gasoline into the high-quality fuel we rely on today.
Overview of Fractional Distillation
Definition and Principle
Definition: Fractional distillation is a separation technique based on boiling point differences, used to divide complex mixtures like crude oil into their individual components.
Principle: The hydrocarbons in crude oil vaporize at different temperatures, allowing them to be separated as they condense at distinct levels in a distillation column. This makes it possible to extract products like gasoline efficiently.
Importance in Petroleum Industry
Purpose: Fractional distillation is essential for refining crude oil into usable products. The process is fundamental for extracting fuels such as gasoline, diesel, and kerosene.
Usage: This method is widely used in refineries around the world due to its reliability and efficiency in separating complex mixtures.
The Fractional Distillation Process
Initial Heating of Crude Oil
Heating: The first step involves heating crude oil in a furnace to approximately 350-400°C (662-752°F). This heat causes lighter hydrocarbons like gasoline to vaporize while leaving heavier components behind.
Objective: The goal is to separate the crude oil into its component parts by causing the lighter hydrocarbons to rise in the distillation column.
Introduction to Fractionating Column
Function: The vaporized crude oil is then introduced into a fractionating column, which is a vertical tower designed to separate different components based on their boiling points.
Design: The column has a temperature gradient, being hotter at the bottom and cooler at the top. This helps in segregating hydrocarbons as they condense at specific points.
Condensation and Separation
Vapor Rising: As the vapor rises through the column, it encounters progressively cooler temperatures. The various hydrocarbons condense at different heights based on their boiling points.
Fraction Collection: Gasoline, which has a boiling range of about 30-200°C (86-392°F), condenses at higher levels in the column, while heavier fractions like diesel settle at lower points.
See also: Which Useful Substances Are Obtained from Crude Oil?
Detailed Steps in the Process
Fractional Distillation Column Operation
Temperature Control: The column’s temperature gradient is carefully controlled to ensure that the hydrocarbons separate efficiently based on their boiling points.
Tray Function: The column contains trays or packing materials that increase the surface area for vapor to condense, which improves the efficiency of the separation process.
Collection of Different Fractions
Gasoline Collection: Gasoline condenses at higher levels in the distillation column due to its relatively low boiling point, and is collected as a liquid.
Other Fractions: Heavier hydrocarbons like kerosene and diesel condense lower in the column, where the temperature is higher. These are collected separately for further refining.
Post-Distillation Processing
Catalytic Reforming
Purpose: Catalytic reforming is a process that enhances the octane rating of gasoline by rearranging its molecular structure.
Process: The gasoline fraction is subjected to high temperatures and pressures in the presence of a catalyst, improving its performance characteristics for use in engines.
Hydroprocessing
Objective: Hydroprocessing removes sulfur and other impurities from gasoline, making it more environmentally friendly.
Method: The gasoline is treated with hydrogen in the presence of a catalyst, which converts sulfur into hydrogen sulfide, an impurity that can be removed.
Blending and Additives
Blending: Gasoline is often blended with additives to enhance its performance, stability, and environmental compliance.
Additives: These additives may include detergents, anti-oxidants, and chemicals that improve fuel efficiency and reduce emissions during combustion.
See also: Where Does Crude Oil That Is Pumped from the Ground Get Processed?
Advantages of Fractional Distillation
Efficiency
Effective Separation: Fractional distillation is efficient because it exploits the distinct boiling points of hydrocarbons, ensuring that valuable components like gasoline are isolated effectively.
High Yield: The process ensures that a significant portion of crude oil is converted into valuable products, reducing waste and maximizing resource use.
Scalability
Industrial Application: The scalability of fractional distillation allows refineries to process large volumes of crude oil, ensuring high production capacity.
Versatility: In addition to gasoline, fractional distillation can be used to separate other hydrocarbon products such as kerosene, diesel, and lubricating oils.
Versatility
Wide Range of Products: Fractional distillation produces a wide range of fuels and chemicals, making it an indispensable method in the energy sector.
Market Flexibility: Refineries can adjust their operations to meet changing demands for different products, making the process highly adaptable.
Conclusion
Fractional distillation remains the most effective method for separating gasoline from crude oil. By utilizing the varying boiling points of hydrocarbons, it allows for precise and efficient extraction of gasoline and other products. This method is widely used in refineries due to its scalability, efficiency, and versatility. Beyond the initial separation, additional processes like catalytic reforming and hydroprocessing ensure that gasoline is of the highest quality, ready for commercial use. Understanding fractional distillation and its associated techniques is crucial for appreciating the complexities of petroleum refining and the production of fuels that power modern life. The widespread use of fractional distillation is a testament to its essential role in the global energy industry, helping to meet the world’s ever-growing demand for fuel and petrochemical products.
Related Topics: